How to Calculate Concentration of a Solution
How to Calculate Molality of a Solution. Its units are moles per liter mol.

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry
For the intended solution preparation purpose It can be taken as approx 37 wt.
. If you solve for M_A you will see that M_A M_BV_B V_A or M_A 10M x 25mL 10 M_A 25M HCl This works because M molesL Note. Whichever method you use once youve determined the expected pH of your solution click below to find the right lab or process electrode for you. Note that with aqueous solutions at room temperature the density of water is approximately 1 kgL so M and m are nearly the same.
Then you can use the following formula to find the concentration. Absorption means that a substance captures and transforms energy while concentration refers to the amount of a substance in a defined space. H increases and hence by the Law of Mass Action the equilibrium is pushed to the left and the concentration of OH- decreases.
So the units for wv concentration are most often given as g100 mL. Concentration of Solution Volume of SoluteVolume of Solution 100. For use in the laboratory the solution must be accurately diluted to the lowest concentration.
Concentration is the number of particles of a compound in a solution relative to the volume of the same solution. The dilution of the solution is a necessary process in the laboratory because the stock solution is usually purchased and stored in a high-concentration form. The pH value is logarithmically and is inversely related to the concentration of hydrogen ions in a solution.
It is the number of moles of target substance solute dissolved in 1 liter of solution. Answer 1 of 6. OD 260 x 002 x dilution factor µgµl Conversion factor for single stranded DNA.
Volume of solvent water 80 mL. This is how concentration of H becomes greater than the concentration of OH-. So volume of solution volume of solute volume of solvent 20 80.
Find expected pH for a given concentration simply by entering the molarity or enter weight and total volume. We can calculate the concentration of a solution in terms of percentage of solute by using the formula. In practice the concentration of an NaOH solution is never determined by calculating it from mass and volume.
M is the mass ie weight of solute that must be dissolved in volume V of solution to make. A commonly accepted concentration for many commercial conc. If the concentration of solution is increased then there are more molecules for the light to hit when it passes through.
Before you calculate the vapor pressure of a mixed liquid you need to identify the substances with which you are working. The normal concentration can be calculated by multiplying the molar concentration by the number of equivalents per mole of solute Equation 4. You do not need to convert volumes.
Some examples are given. The pH to H formula that represents this relation is. So if you have a compound that dissociates into cations and anions the minimum concentration of each of those two products will be equal to the concentration of the original compound.
The molar concentration unit mol L M is a conventionally widely used as concentration method. See Tables 1 and 2 for some typical values for the. It takes 25mL of NaOH to neutralize the acid.
Here is how to calculate the concentration. In these cases you can easily calculate the primer concentration from an OD 260 reading. This worked example problem illustrates the steps to calculate the concentration of ions in an aqueous solution in terms of molarity.
You want a volume of your solute and a volume of your solution or a mass of both. The first thing you need to do is get a measurement of the amount of solute or substance youre interested in then get the same type of measurement for the whole solution eg. Molarity is the number of moles of a substance per unit volume.
You can even calculate the impact of dilution entering starting molarity and final volume. The concentration of ions in solution depends on the mole ratio between the dissolved substance and the cations and anions it forms in solution. C is the desired concentration of the final solution with the concentration unit expressed in units of activity per volume of solution eg UnitsmL.
Instant free online tool for partmillion ppm to gramliter conversion or vice versa. Therefore the units for wv mv concentration are grams of solute per 100 mL of solution g100 mL. This means that the weightvolume percentage concentration massvolume percentage concentration can be given in different but equivalent ways.
Calculate the molality of the solution. It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidicbasic. In the above example.
The concentration can be calculated using the following formulas. Molar concentration which is also called molarity denotes the number of moles of dissolved compound per liter of solution. Also explore tools to convert partmillion ppm or gramliter to other concentration - solution units or learn more about concentration - solution conversions.
As a reminder a solution is formed when a solute is dissolved in a solvent the chemical that dissolves is always the solute and the chemical that does the dissolving is always the solvent. For pH you have to use molar concentration for the formula to work out. Calculate the molality of the solution.
Supplied by a variety of brands. Thats because NaOH cant be bought chemically pure and because its so hygroscopic that its mass will visibly increase while it. Dilution can also be achieved by mixing a higher concentration solution with the identical solution of lower concentration.
Heres how that works. The pH scale pH is a numeric scale used to define how acidic or basic an aqueous solution is. Molarity is one of the most common units of concentration.
Volume of solute alcohol 20 mL. Formula to calculate concentration from absorbance. The partmillion ppm to gramliter gL conversion table and conversion steps are also listed.
A is the activity of the solid material with the activity unit expressed in units of activity per mass of material eg Unitsmg. M_AV_A M_BV_B Lets assume you are titrating a strong acid 10 mL unknown concentration HCl with a strong base 10 M NaOH. Your attempt 2 is.
A solution is prepared by dissolving 4 g of NaOH to give 5 0 0 m l of it. Molality is used to express the concentration of a solution when you are performing experiments that involve temperature changes or are working with colligative properties. So you can in fact take H concentration as 10-ph which gives the total concentration of H due to both acid and water.
The normal concentration of a solution normality C N is always equal to or greater than the molar concentration molarity C M of the solution. Lets assume that we dilute the primer from above 1200 and the OD260 reading was 0132.

5 Easy Ways To Calculate The Concentration Of A Solution Chemistry Study Guide Ap Chemistry Solutions

How To Calculate The Concentration Of A Solution Solution Chimie

Calculate Concentration Acids Alkalis Problems 7 School Study Tips Concentration Chemistry

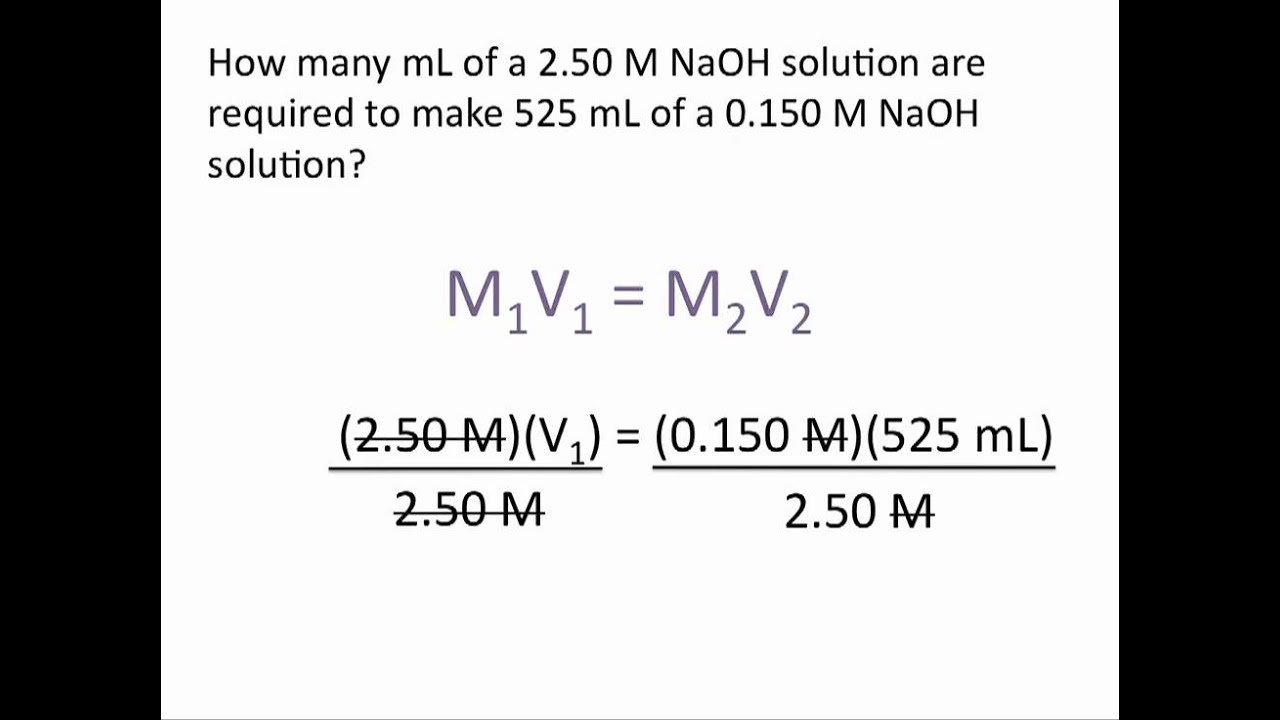
Dilution Problems Chemistry Tutorial Chemistry Notes Chemistry Microbiology
0 Response to "How to Calculate Concentration of a Solution"
Post a Comment